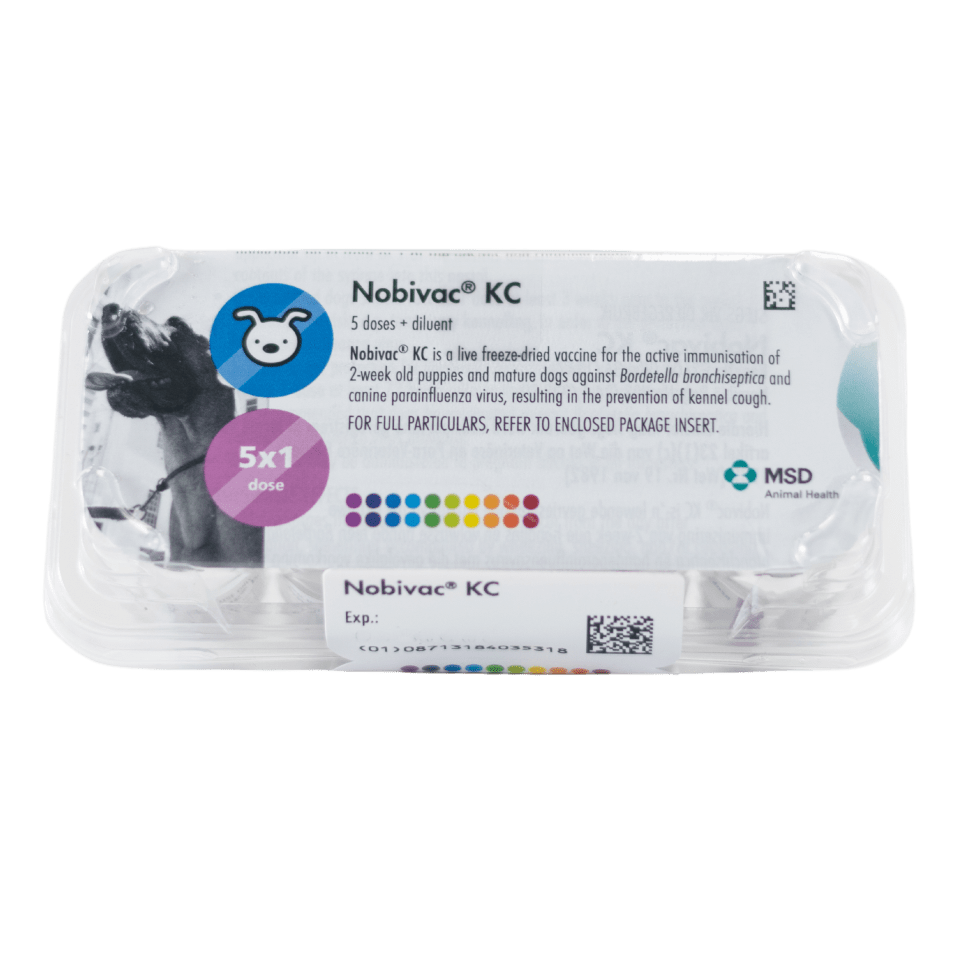
Nobivac® KC
NOBIVAC® KC is a live freeze-dried vaccine for the active immunisation of 2-week-old puppies and mature dogs against Bordetella bronchiseptica and canine parainfluenza virus, resulting in the prevention of kennel cough. Immunity is complete 72 hours after vaccination.
NOBIVAC® KC
Reg. No. G2604 (Act 36/1947)
Namibia Reg. No. V03/24.1/928 [NS2]
Only for use by or under the supervision of persons registered in terms of, or authorised in terms of, Section 23 (1) (c) of the Veterinary and Para-Veterinary Professions Act, 1982 (Act 19 of 1982).
View the video above with Dr Steven Wimberley from Westville Veterinary Hospital on how to administer the Nobivac® KC vaccine.
INDICATIONS
Nobivac® KC is a live freeze-dried vaccine for the active immunisation of 2-week-old puppies and mature dogs against Bordetella bronchiseptica and canine parainfluenza virus, resulting in the prevention of kennel cough. Immunity is complete against B. bronchiseptica after 72 hours, and against canine parainfluenza virus 3 weeks after vaccination.
Nobivac® KC induces at least 1 year (56 weeks) protection against clinical signs, as well as infection against Bordetella bronchiseptica and canine parainfluenza virus.
TARGET SPECIES
Dogs
COMPOSITION
Each dose of Nobivac® KC contains ≥ 108,3 cfu of Bordetella bronchiseptica strain B-C2 and ≥ 103,8 TCID50 of canine parainfluenza virus strain Cornell. The freeze-dried vaccine is reconstituted with the sterile diluent provided.
STORAGE INSTRUCTIONS
- Store between 2 °C to 8 °C in a refrigerator.
- Do not freeze.
- Store in the original package in order to protect from light.
- Avoid prolonged or repetitive exposure to high ambient temperatures (20 °C to 25 °C) following withdrawal from the refrigerator prior to use.
- Do not use this veterinary medicinal product after the expiry date which is stated on the container label.
- Diluent: Store at room temperature below 25 °C.
- The reconstituted vaccine should be used within 1 hour.
WARNINGS
- Vaccinate healthy puppies and dogs only.
- Do not open and reconstitute the vaccine until ready to start the vaccination procedure.
- Use the vaccine immediately after reconstitution.
- Do not mix Nobivac® KC with other vaccines or other products.
- Do not administer in conjunction with other intranasal treatments or during antibiotic treatment.
- Use according to the number of doses as indicated on the label.
- Do not over- or under-dose the vaccine.
- Do not use within 2 weeks of antimicrobial treatment.
- KEEP OUT OF REACH OF CHILDREN, UNINFORMED PERSONS AND ANIMALS.
- Although this vaccine has been extensively tested under a large variety of conditions, failure thereof may ensue as a result of a wide range of reasons. If this is suspected, seek veterinary advice and notify the registration holder.
PRECAUTIONS
- Observe aseptic precautions. Ensure that all vaccination equipment (containers, syringes and needles) is clean and sterile prior to and during use. Use sterile equipment when administering the vaccine.
- As with all vaccines hypersensitivity or anaphylactic reactions may occur.
- Do not use disinfectants or antiseptics to sterilise any equipment.
- It is good vaccination practice when handling the vaccine to avoid contact with the eyes, hands and clothing.
- Adhere to the vaccination programme to obtain optimum results.
- Do not eat, drink or smoke whilst handling the product.
- Destroy any unused vaccine and dispose of all the empty vaccine containers and disposable equipment after use in accordance with National Environmental Management: Waste Act, 2008 (Act No. 59 of 2008).
- Do not contaminate rivers, dams or any water sources with containers or waste.
DIRECTIONS FOR USE – USE ONLY AS DIRECTED
Shake well after the addition of the diluent.
Dosage and administration
- Administer into only 1 nostril of the puppy or dog.
- Allow the diluent to reach room temperature, below 25 °C.
- Aseptically reconstitute the freeze-dried pellet with the diluent provided.
- Shake well after the addition of the diluent. The contents of the vial must be used within 1 hour after reconstitution of the vaccine.
- Fill a 1 mℓ syringe with 1 dose (0,4 mℓ) of vaccine. Remove the needle and connect the supplied applicator tip.
- Fix the head of the puppy or dog in a normal upright position, place the applicator tip in front of 1 of the nostrils and carefully administer the entire contents of the syringe into this nostril.
- Unvaccinated dogs should receive 1 dose at least 3 weeks prior to the period of anticipated risk, e.g. temporary kennelling, in order to get protection from canine parainfluenza virus.
- In order to get protection for Bordetella bronchiseptica, unvaccinated dogs should receive 1 dose at least 72 hours prior to the period of anticipated risk.
- It is recommended that dogs be vaccinated against Bordetella bronchiseptica and canine parainfluenza virus annually.
- Nobivac® KC may be administered to pregnant bitches in all trimesters.
SIDE EFFECTS
- Mild discharges from the eyes and nose can occur from the day after vaccination, sometimes accompanied by wheezing, sneezing, and/or coughing, particularly in very young susceptible puppies.
- Signs are generally transient, but in occasional cases may persist for up to 4 weeks.
- In animals, which show more severe signs, appropriate antibiotic treatment may be indicated.
OVERDOSE
- In very young puppies, signs of upper respiratory tract disease may occur after vaccination, including ocular and nasal discharges, pharyngitis, sneezing and coughing.
- In animals that show more severe signs, appropriate antibiotic treatment may be indicated.
PRESENTATION
Polyethylene terephthalate (PET) tray containing 5 single dose vials of freeze-dried vaccine with 5 vials of sterile diluent and applicators, or 3 mℓ (single dose) or 10 mℓ (5 and 10 dose) with a vial of sterile diluent and applicator.
REGISTRATION HOLDER
Intervet South Africa (Pty) Ltd.
20 Spartan Road,
Spartan, 1619,
RSA
Tel: +27 (0) 11 923 9300
E-mail: msdahza@msd.com
www.msd-animal-health.co.za
DATE OF PUBLICATION OF THIS PACKAGE INSERT 22 January 2016
